Abstract
BACKGROUND: GRFS is a composite endpoint developed by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) to reflect the major outcomes post HCT. Events in GRFS include grade 3-4 acute GVHD (aGVHD 3-4), systemic therapy-requiring chronic GVHD (cGVHD), relapse, or death. We reported that bone marrow (BM) grafts from matched sibling donor (MSD) led to best GRFS at 1-and 2-years compared with peripheral blood (PB) grafts from any donor or umbilical cord blood (UCB). (Holtan et. al. Blood 2015 & Mehta et.al. Haematologica. 2016) These studies excluded haploidentical (haplo) and mismatched (MM)-HCT.
Here, we analyzed GRFS and chronic GVHD-free relapse-free survival (CRFS) among alternative donor HCT (UCBT and one-antigen MM (7/8)-BM HCT) for pediatric patients who did not have a MSD or matched unrelated donor. However, because too few patients received 7/8-PB or haplo with post HCT cyclophosphamide, these were excluded.
METHODS: Data were obtained from the CIBMTR. Patients <18 years of age with acute myeloid leukemia or acute lymphoblastic leukemia (ALL) in remission who received UCBT or 7/8-BM HCT between 2000 and 2014 after myeloablative conditioning were included. Exclusion criteria were prior autologous or allogeneic HCT, ex-vivo T-cell depletion or CD34 selection and those with UCB unit <4/6 HLA. Multivariable analysis was done using Cox's proportional hazards (PH) modeling with time-dependent adjustments as needed for stepwise modeling, selecting factors using a threshold of 0.05 for both entry and retention in the model. CRFS was defined as absence of cGVHD, relapse and death.
RESULTS: 1613 patients were analyzed, including UCBT (n=1441) and 7/8-BM (n=172). Overall, most patients had ALL (63%), 64% received total body irradiation based conditioning, about 60% received ATG or alemtuzumab and 86% UCBT used a single unit. Methotrexate based GVHD prophylaxis was more commonly in 7/8-BM (79%) than in UCBT (15%). Other characteristics were similar in two groups. Median follow-up was 71 months in UCBT and 96 months in 7/8-BM groups.
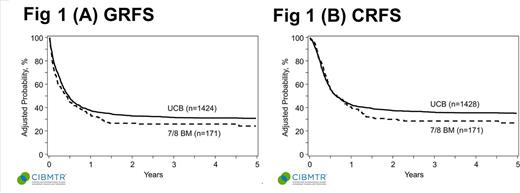
In multivariate analysis, 7/8-BM had similar GRFS (HR 1.19, p=0.1) and CRFS (HR 1.11, p=0.36) as UCBT [Fig 1a & 1b]. Risks of aGVHD 3-4 (HR 1.68, p=0.007) at any time and cGVHD (HR 7.11, p<0.0001) beyond 14 months, but not before, were significantly higher in 7/8-BM than UCBT. 7/8-BM group had similar risk of relapse (HR 1.26, p=0.11) as UCBT. Disease free survival (DFS; HR 0.87, p=0.4) was similar in both groups up to 14 months beyond which 7/8-BM had inferior DFS (HR 2.33, p=0.0008). Overall survival (OS; HR 1.08, p=0.6) was similar in both groups.
CONCLUSION: UCBT and 7/8 BM groups yield similar long term morbidity and mortality as measured by GRFS and CRFS, but 7/8-BM had higher risk of aGVHD 3-4, late cGVHD, and inferior late DFS. No preference of alternative donor source can be made; based upon this analysis, both are acceptable options. Improvements in GVHD control could broaden the applicability of partially matched BM grafts.
Holtan: Incyte: Other: One-time advisory board member. Arora: Takeda Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal